* Question
What is a three-electrode system in electrochemical measurement?
* Answer
A three-electrode system is a standard configuration used in electrochemical measurements to accurately control and monitor the potential and current during a reaction.
It separates the roles of current flow and potential measurement, providing higher precision and stability compared to a simple two-electrode setup.
This system is fundamental in research and industrial applications such as battery testing, corrosion analysis, and electrochemical sensor development.
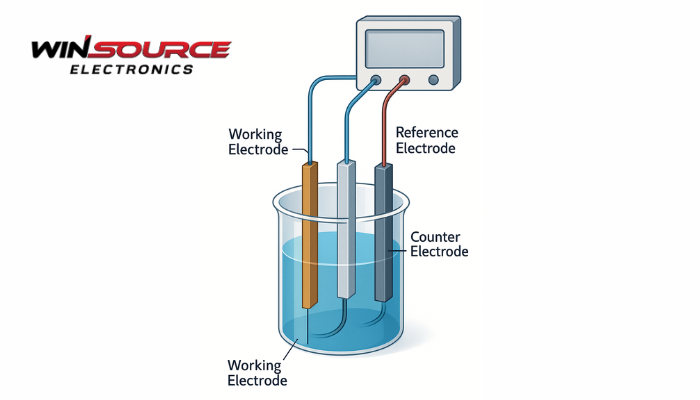
1. Components of a Three-Electrode System
A typical three-electrode setup includes:
- Working Electrode (WE):
The electrode where the electrochemical reaction of interest occurs—such as oxidation or reduction.
Examples: platinum, gold, glassy carbon, or modified semiconductor electrodes. - Reference Electrode (RE):
Provides a stable and known potentialagainst which the working electrode’s potential is measured.
It does not pass significant current.
Common examples include Ag/AgCl and Saturated Calomel Electrode (SCE). - Counter (Auxiliary) Electrode (CE):
Completes the circuit by allowing current to flow between the working electrode and itself.
It typically has a large surface areato minimize polarization, often made of inert materials like platinum wire or graphite.
2. Working Principle
During measurement, a potentiostat controls the potential difference between the working electrode and reference electrode while measuring the resulting current that flows between the working and counter electrodes.
This arrangement enables independent and accurate control of potential—essential for techniques like cyclic voltammetry, chronoamperometry, and electrochemical impedance spectroscopy (EIS).
3. Advantages of the Three-Electrode Configuration
- High precision:Separating current and potential paths eliminates interference between them.
- Stable reference:The constant potential of the reference electrode ensures reproducible measurements.
- Wide applicability:Suitable for studying redox reactions, corrosion rates, material coatings, and electrode kinetics.
4. Applications
The three-electrode system is foundational in:
- Battery R&D– to study electrode performance under controlled conditions.
- Fuel cell and supercapacitor testing– for analyzing reaction kinetics.
- Corrosion science– to monitor passivation and degradation processes.
- Biosensors and electrochemical sensors– for selective detection of chemical species.
Summary Insight
In summary, a three-electrode system provides a controlled and precise framework for studying electrochemical reactions by isolating voltage measurement from current flow.
It is the backbone of modern electrochemical research and sensor development, enabling scientists and engineers to analyze reaction mechanisms, material stability, and electrode performance with high accuracy.

COMMENTS